
Two case studies illustrate a systematic approach.

Two case studies illustrate a systematic approach.

The much needed modernization of the agency's IT systems and inspection capabilities will likely fall prey to budget shortfalls.

The use of disposables has changed significantly in the biopharmaceutical industry.

Both innovator and generics companies are using analytics to support comparability arguments.

In our increasingly global industry; foreign manufacturing inspections are more important than ever.

Best practices from Big Biotech, including how to handle new product introductions.
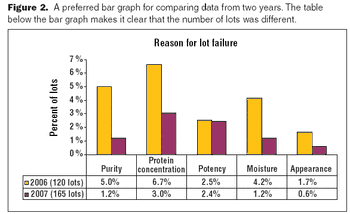
The key to a good graphical presentation is to select the method that best fits the data.
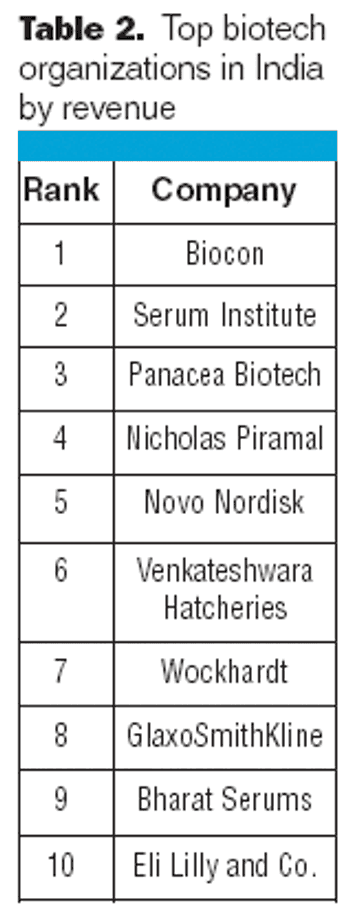
Indian biogenerics could form a major piece of the global biotherapeutics market in the future.

Putting business principles to work in biotechs requires careful implementation of nine critical business systems.