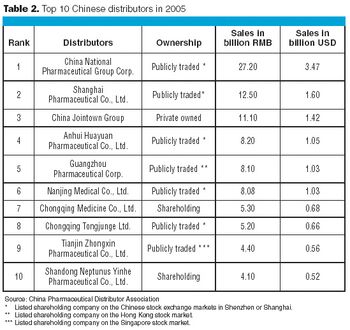
China today represents a $15-billion market for pharmaceutical products. China's pharmaceutical industry has been expanding at about 20% over the past five years. It has been predicted that China will become the world's fifth largest single pharmaceutical market by 2010. With such a fast-growing market segment and a huge population, simply getting pharmaceuticals to the patients and healthcare providers is becoming a daunting task. Biopharmaceuticals distributers face the same challenge, with the added complications associated with cold-chain management, shelf-life, and product stability.