
SAFC Pharma has announced the completion of an addition to its Carlsbad, CA, facility, which specializes in the process development and manufacturing of viral vaccines and viral therapeutics.
Chitra Sethi, Managing Editor, BioPharm International

SAFC Pharma has announced the completion of an addition to its Carlsbad, CA, facility, which specializes in the process development and manufacturing of viral vaccines and viral therapeutics.

Colorado-based InVitria, a division of the biopharmaceutical company Ventria Bioscience, has launched an animal-free recombinant transferrin for use in mammalian cell culture.

Protalix BioTherapeutics, Inc. (Carmiel, Israel) gained orphan drug status from the US FDA for prGCD, a development drug for Gaucher?s disease, on September 9. The orphan drug designation for prGCD was granted by the FDA?s Office of Orphan Products Development and comes less than a month after the drug received fast-track designation from the FDA.

Shire plc (Cambridge, MA) has completed its submission of a new drug application (NDA) for velaglucerase alfa, its enzyme replacement therapy in development for the treatment of Type 1 Gaucher disease, with the US Food and Drug Administration.

Pharmaceutical giant Pfizer, Inc., (New York, NY) has opened a new 64,600-square-foot biotech plant in Strangnas, Sweden.

London-based GlaxoSmithKline?s Hiberix, a haemophilus influenzae Type b (Hib) vaccine, was approved by the US Food and Drug Administration.

The House Energy and Commerce Committee approved a legislative amendment that would give 12-years data exclusivity to innovator biologics. The amendment was introduced by Reps. Anna Eshoo (D-CA) and Jay Inslee (D-DC).

French drug manufacturer Sanofi Aventis will purchase the Indian vaccine company Shantha Biotechnics for $784 million.

In an effort to boost its biologics pipeline, Bristol-Myers Squibb (BMS, New York, NY) will acquire Medarex (Princeton, NJ) for approximately $2.4 billion.

The Senate?s Health, Education, Labor, and Pensions Committee yesterday passed The Affordable Health Choices Act, the committee?s healthcare reform legislation that gives 12 years of data exclusivity to innovator biologics. The committee had adopted the 12-year data protection amendment to the healthcare legislation on Monday.

When Roche bought Genentech in March, layoffs were expected, and now the company has begun downsizing at Genentech?s South San Francisco headquarters.

The newly formed Rx-360 consortium, an international consortium developed by members of the pharmaceutical and biotech industries aimed at improving global supply chain security, had an impressive turnout of over 125 people at its launch meeting in Washington, DC, on June 5. The objective of the launch meeting was to increase awareness, solicit membership, and pressure-test shared audit models.

In advance of the Senate Health, Education, Labor, and Pensions Committee?s meeting to be held on Friday to consider amendments to the healthcare reform bill, including several amendments related to biosimilars, Biotechnology Industry Organization?s (BIO) President and CEO Jim Greenwood reaffirmed BIO?s support for a 12-year data exclusivity period for biologics.

Genzyme has voluntarily recalled all remaining stock of Fabrazyme 5 mg (agalsidase beta), with batch numbers A8049H04 and A8060H01.

OctoPlus has started production in its new manufacturing facility in Leiden.

Vetter Pharma International (Ravensburg, Germany) has completed the installation of six automatic packaging lines at Vetter Secondary Packaging (VSP), the company?s new packaging services facility.

The newly formed Rx-360 consortium had an impressive turnout of over 125 people at its launch meeting in Washington, DC, on June 5.

A new report by FTC, entitled ?Follow-on Biologic Drug Competition,? examines whether the price of biologics is likely to be reduced by competition from FOBs.

ImClone Systems, Inc. (Branchburg, NJ), and Bristol-Myers Squibb (BMS, New York, NY) voluntarily recalled 13 lots of their cancer drug Erbitux (cetuximab) after a report from a doctor?s office about a leaking cap on a vial of the biologic.
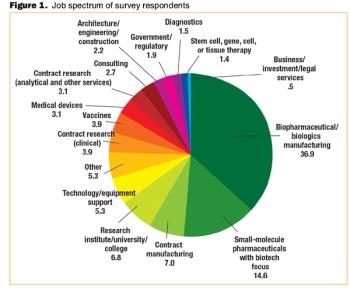
Will the global economic crisis affect your job? BioPharm International's third annual salary survey finds out.

Eli Lilly acquires Imclone

Eli Lilly (Indianapolis, In) is the mystery bidder "in advanced talks" to acquire ImClone Systems (New York, NY).

Biotechnology company ImClone Systems (New York, NY) has rejected the offer it received last month from its cancer drug partner Bristol-Myers (BMS, New York, NY) to acquire ImClone for $60 per share.

Bristol-Myers Squibb has proposed to acquire its cancer drug partner ImClone Systems in an all-cash deal worth around $4.5 billion.

The FDA has ordered Amgen and Johnson & Johnson to make safety-related changes to the labeling of their erythropoeisis-stimulating agents (ESAs), Aransesp and Procrit.

Genentech, Inc., (South San Francisco, CA) has formed a special committee of its Board of Directors to assess the proposal from the Swiss drug-maker Roche (Basel, Switzerland) to take over the remaining shares of the American biotech giant.

Health and Human Services Secretary Mike Leavitt has announced that his agency is amending its budget request for FY 2009 to include an additional $275 million for the FDA.

US Food and Drug Administration's Division of Biologic Oncology Products has approved two new biologics license application (BLA) supplements expanding the approval of Genentech's Herceptin (trastuzumab) for the treatment of breast cancer.

The US FDA has embarked on a multiyear initiative to hire hundreds of individuals with science and medical backgrounds to help meet the agency?s responsibilities to ensure the safety and efficacy of human and veterinary drugs, biological products, medical devices, food, and cosmetics.

Takeda Pharmaceutical Company Limited (Osaka, Japan) will acquire Millennium Pharmaceuticals, Inc. (Cambridge, MA), for approximately $8.8 billion through a cash tender offer of $25.00 per share.