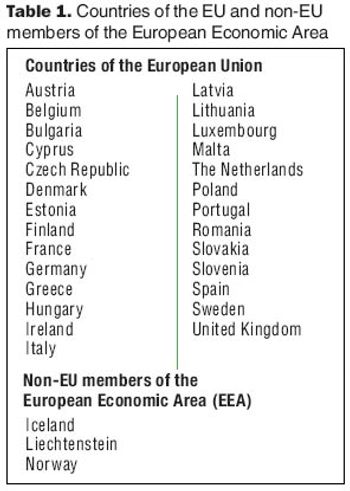
A good understanding of European regulations governing batch release testing will facilitate your collaboration with a contract laboratory.

A good understanding of European regulations governing batch release testing will facilitate your collaboration with a contract laboratory.
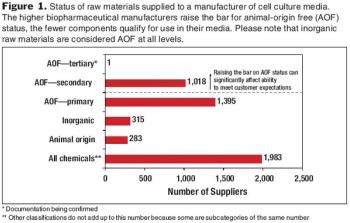
How to successfully balance patient safety with supply-chain management
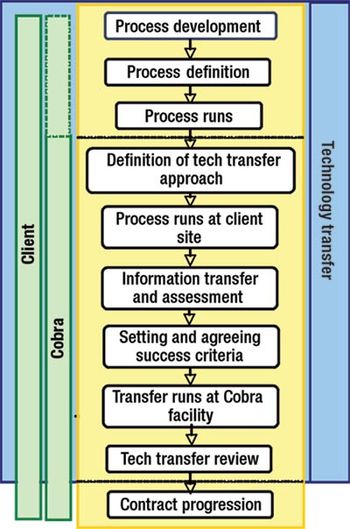
Effective tech transfer can save time and effort in later manufacturing processes.

Outsourcing introduces considerable complexity into ensuring compliance with good manufacturing practices. This article offers FDA guidance for how to ensure compliance in the outsourcing environment.

Communication between contract analytical laboratory and client is extremley important when out-of-specification (OOS) results arise.