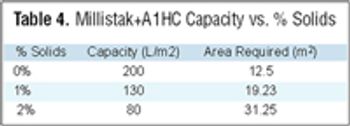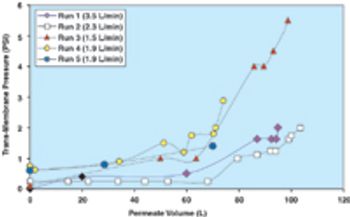
Filtration is one of the most commonly used unit operations in the manufacturing of biopharmaceuticals. This is the second part of the fourth article in the "Elements of Biopharmaceutical Production" series. In this second segment, Manoj Menon and Frank Riske present an approach for the development and optimization of a TFF application, followed by a contribution from Jennifer Campbell and Elizabeth Goodrich reviewing key issues involved in validation of a TFF step.