
John Ward, vice-president of Engineering at Patheon, discusses the potential for cross contamination in the manufacture of biopharmaceuticals.

John Ward, vice-president of Engineering at Patheon, discusses the potential for cross contamination in the manufacture of biopharmaceuticals.

Andrew Bulpin, head of Process Solutions Strategic Marketing & Innovation at MilliporeSigma spoke with BioPharm International about the requirements of expression systems in biosimilar development.

The baculovirus-insect cell system can produce large quantities of complex protein in a short period of time.

Zeiss’s Axio Observer inverted microscope platform includes new automation features, allowing researchers to perform multimodal imaging of living and fixed specimens.

The Prominence Ultra-Fast Preparative and Purification Liquid Chromatograph (UFPLC) from Shimadzu enables fast recovery of highly purified target compounds from complex samples including organic synthesis reaction mixtures and natural products.

A novel coiled flow inversion reactor (CFIR) improves process productivity and performance.

Biopharma must see regulators as partners in their efforts to provide safe and effective therapies.

What will manufacturers have to do to ensure the continued uptake of biosimilars?

Republican control of Washington promises overhaul of healthcare and medical product regulation.

This report describes the first known attempt at quantifying the success of such processes in inducing nucleation on a 56–m2 freeze dryer operating at a load of 195,960 vials.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.

Rookie API developers beat pharma at its own game.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.
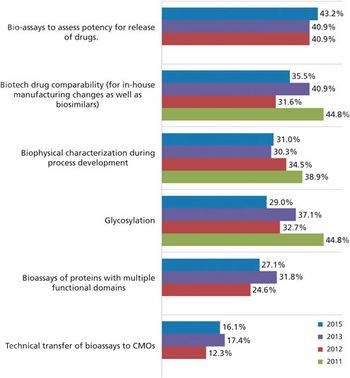
Biosimilars may be the key to CMO growth.

Job security may have increased, but biopharma professionals remain somewhat dissatisfied with pay levels.

A consistent approach in assessing risk is an important aspect of successful quality management.

Click the title above to open the BioPharm International December 2016 issue in an interactive PDF format.