
Reliable, high-quality products require innovative analytics and production.

Reliable, high-quality products require innovative analytics and production.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to prepare for an inspection by a foreign regulatory agency.
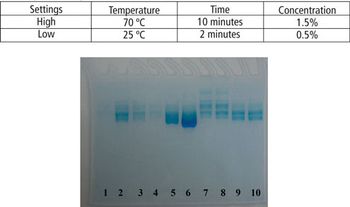
This article demonstrates that a modified SDS–PAGE can be easily used as a tool for quantifying the degree of protein degradation.

New data analytics tools help solve complex problems in a biotherapeutic development process.
In addition to having the optimal cell line and process, it is crucial to have the optimal cell culture medium and feed to maximize performance potential.
Advances in technology are increasing the productivity and efficiency of commercial-scale chromatography bioprocesses.

To investigate the best culture conditions, the authors used response surface methodology via Box-Behnken design.

CMO executives are focusing on M&A activity, new business models, and fundraising limits.
The development of mAb formulations poses challenges at the manufacturing, stability, analytical, and administration levels.

Vendor selection and materials testing are complex enough, but in today’s volatile environment, risk mapping and monitoring are also crucial.

Interactions between biologic drug products and the components of prefilled syringes can cause protein aggregation, but there are alternative materials that can help mitigate this problem.

Click the title above to open the BioPharm International November 2016 issue in an interactive PDF format.