
Acquisition binges often lead to hangovers; here’s what to watch out for.

Acquisition binges often lead to hangovers; here’s what to watch out for.
Scale-up of complex, innovative products requires commercialization models that are sustainable.
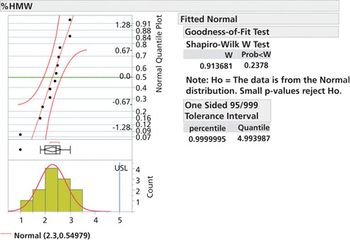
Specification limits should be set early in drug development and refined in later phases as data becomes available.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on data integrity.

An understanding of continuous process validation can lead the way to consistent approaches, reduced investigation times and observations, the avoidance of lost batches, and high-quality products.

Barriers to the production of biopharmaceuticals in moss are explored.

Advances in single-use systems, consumables, and continuous manufacturing show steady progress.

Advances in cell line engineering, process optimization, and in-vitro glycosylation are making a difference.

FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

More efforts are needed to raise awareness of biosimilars among physicians and patients in Europe and address scepticisms about the quality and safety of biosimilars.

The Cadence Acoustic Separator (CAS) from Pall Life Sciences enables the purification of bioprocesses fluids without the use of primary depth filters or centrifuges.
The ArcAir communication package from Hamilton Company enables Bluetooth 4.0 wireless connectivity in all environments.

Click the title above to open the BioPharm International June 2016 issue in an interactive PDF format.

Immuno-oncology drugs are demonstrating patient benefits, but growing resistant to the high cost has implications for patients, market access, and manufacturers.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.