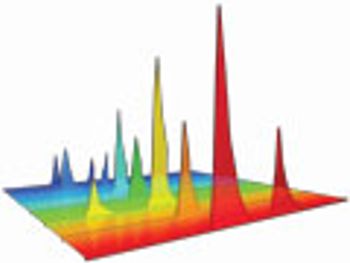
Characterization of stability performance provides a clear, statistically defendable method for determining accelerated stability.

Characterization of stability performance provides a clear, statistically defendable method for determining accelerated stability.

New formulations and expanded vaccine production are encouraged.

Progress is being made in the development of harmonized best practices for single-use systems.

New approaches to vaccine production are targeting rapid supply for pandemic situations and broadly effective therapeutic treatments.

Clinical Syringe Packages Increase Productivity

Industry players offer suggestions for quality metrics as FDA continues to try and solve the problem of drug shortages.

New human and plant-based expressions systems can enable faster product development and improve quality at potentially lower costs.

Integrated Single-Use Sterile and SIP Connector Provide Flexibility
Review regulatory requirements and the use of viral-challenge studies in drug development.

Biopharma companies should not overlook India's growing market.

The CMO industry's value proposition is limiting its market penetration.

Changes are needed to maintain US biopharma innovation leadership.

Aseptic Valve Range Increases Sterility

Click the title above to open the BioPharm International May 2014 issue in an interactive PDF format.