
This article presents first-hand perspectives from industry users to suppliers of single-use sensors.

This article presents first-hand perspectives from industry users to suppliers of single-use sensors.

More reliable operations would accelerate product development and prevent drug shortages.
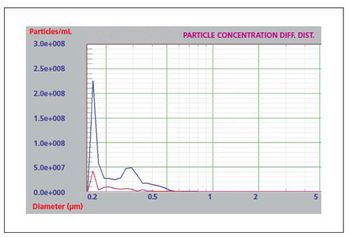
Liquid particle counters are ideal for protein aggregation studies.

Ethylene vinyl acetate (EVA) drug-release technologies are explored.
Second-generation needle-free injection systems will make parenteral drug administration more convenient, efficient, and safe.
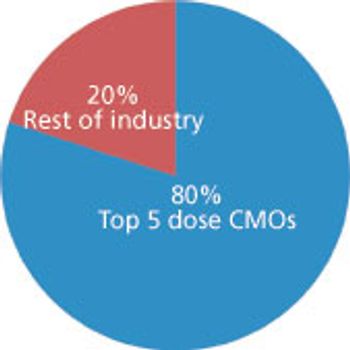
Big service providers get bigger faster thanks to Big Pharma.

jayk7/Getty Images

Phenomenex’s Kinetex F5 is a pentafluorophenyl propyl core-shell phase column designed to improve reproducibility and reduce method development time.
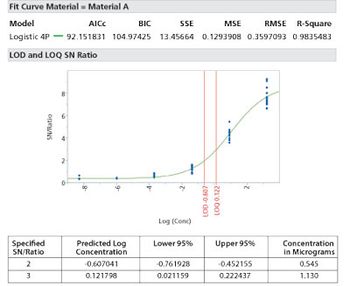
Care needs to be made to match the method of limit determination to the analytical method.

Bausch’s second generation Type 529 is a semi-automatic IV bag filling and closing machine designed for aseptic packaging of infusions, parenteral drugs, and other fluids with a fully integrated automatic closing system to eliminate possible contamination.

FDA approves a biosimilar and loses a commissioner in March.

Click the title above to open the BioPharm International April 2015 issue in an interactive PDF format.