The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.

The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.
Rapid methods to test CAR-T therapies for potential contamination are on the horizon.

FDA and industry see progress and challenges in bringing cutting-edge medicines to patients.

Heightened global uncertainty could slow bio/pharma development activity.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.

Report: Biologics contribute to rebirth of biopharma innovation.

Understanding of the risks associated with FMEA is crucial in lot release testing.
The recognition that microbial artifacts are capable of modulating the mammalian immune system is an emerging view of biologic drug contamination control testing.

The authors describe the impact of the knocking of the pgi gene of the wild type MG1655 strain on the growth kinetics of plasmid-free and plasmid-bearing cells.
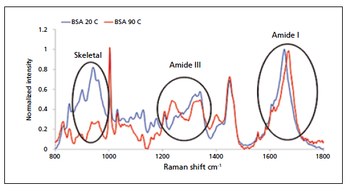
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

Click the title above to open the BioPharm International February 2016 issue in an interactive PDF format.