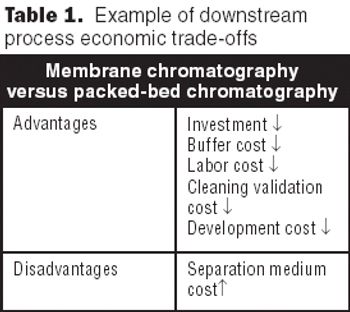
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.

The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
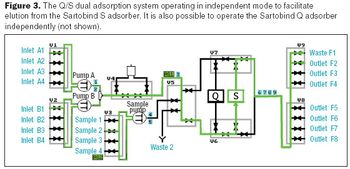
A membrane adsorber in retention mode can be used efficiently in a commercial antibody manufacturing process.
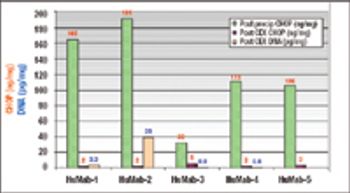
Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.
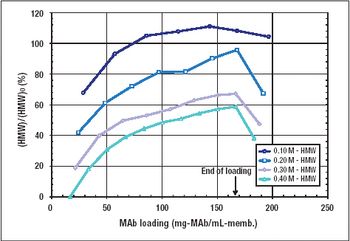
This alternative to column chromatography is suitable for flow-through as well as bind-and-elute purification operations.

New techniques can overcome bottlenecks in existing facilities.
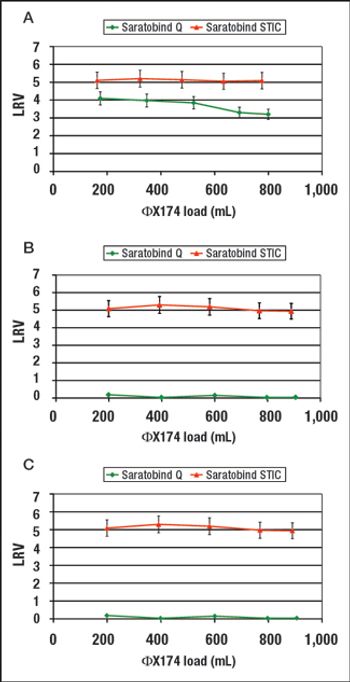
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.
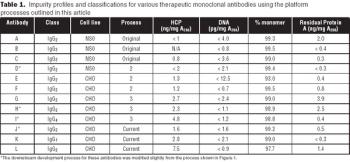
A purification scheme to maximize the efficiency of the purification process and product purity while minimizing the development time for early-phase therapeutic antibodies.