
Biosimilars : New Guidelines Set for Monoclonal Antibodies

Biosimilars : New Guidelines Set for Monoclonal Antibodies

Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?
Scaling up stem-cell cultures requires careful consideration of the bioreactor design.

The procurement organization rethinks sourcing for maximum efficiency and results.

NIBRT's Jayne Telford provides an overview of biopharmaceutical analytics and their accompanying qualification and validation steps.
This month, Jerold Martin of Pall Life Sciences takes a look at protein recovery through direct-flow microporous membrane filters over the past 25 years.

A 10-step systematic approach to analytical method development and validation can improve the quality of drug development.

Creating an effective nucleic acid-based vaccine requires protecting the fragile nucleic acid from degradation, effective transfection of the targeted cells, and producing high enough levels of antigen to evoke a robust immune response.
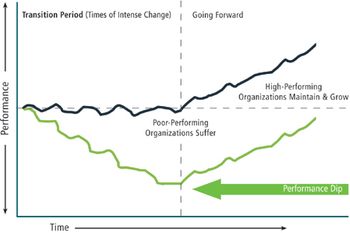
A disciplined approach to changing behavior can achieve change agility.

The author considers the types of tubing available to the industry and how to make an informed selection.

Manufacturing and in-depth characterization provide basis for demonstrating product equivalence.

The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on mAbs.

Patrick Jackson of Vindon Scientific offers key considerations for choosing an outsourced sample storage facility.

The government of Turkey is drawing up a program in coordination with the pharmaceutical industry to create ways to make the country a regional production center for pharmaceuticals serving Europe, Central Asia, and the Middle East.

Global coordination tactics that incorporate online technologies and social media are reshaping disease prevention and response.

Understanding overall supplier capability versus the critical-to-quality attributes of your product can reduce both risk and cost.

This article discusses the evaluation of a novel single-use fluidized bed centrifuge for harvesting of antibodies.

Scaling up stem-cell cultures requires careful consideration of the bioreactor design.