
Vaccine Packaging and Delivery Systems

Vaccine Packaging and Delivery Systems

India has enforced stricter patent laws compared to other countries. For a drug to be patentable in India, the invention has to be novel, inventive, and industrially applicable.

The manufacturing capacity-sharing model between Merck and MedImmune ushers in a new paradigm of "co-opetition."

Do you have to worry about FDA releasing confidential data? Apparently so.

User fees aim to speed approvals and support inspections.

Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.

Glenn Thorpe of West Pharmaceutical Services gives an update on packaging and delivery methods for vaccines.

Measuring the size of the market for contract manufacturing services requires a careful hand.

A new report highlights the industry's contributions to neglected diseases and calls for further collaboration.

A handful of therapeutics have performed extremely well in 2012, but as a whole, life-sciences are still down from 2010.
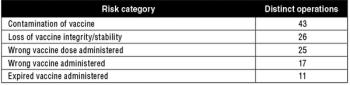
Packaging vaccines using prefilled syringes can increase dosing efficiency, reduce costs, and improve patient safety. This article is part of a special section on vaccines.
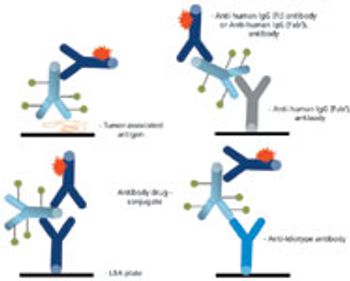
The complex structure of ADCs necessitates different analytical strategies than those for either small molecules or unconjugated monoclonal antibodies.

NIBRT's Ian Nelligan on what to expect when starting a downstream process.
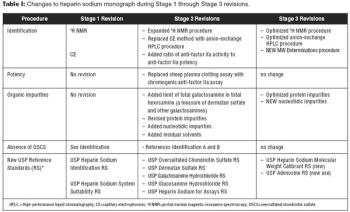
USP optimizes identification tests and impurities procedures.
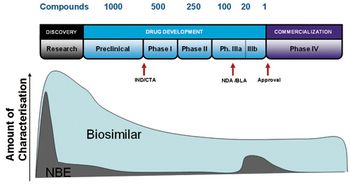
Understanding opportunities and challenges across all major phases of development.

Working together affords many unseen opportunities for pharmaceutical innovation.

Will the next US President support the backbone of our industry?

Marco Chacon of Paragon Bioservices discusses the challenges associated with outsourced vaccine manufacturing.
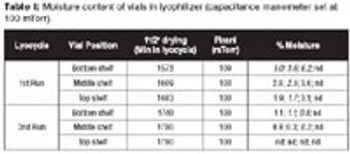
Using an alternate moisture-generation method may provide more accurate data for regulatory submissions.
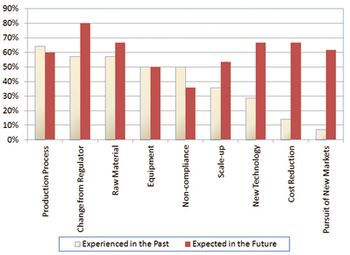
MIT survey results address product and site characteristics that statistically correlate with quality performance.

Anne Marie Dixon of Cleanroom Management Associates, gives us an update on her 1988 article, "Clean Room Management."