
A New Era in Cell Culture Media Development

A New Era in Cell Culture Media Development

Steven S. Kuwahara, PhD, principal consultant at GXP BioTechnology LLC, gives an update on "Engineering the Cell-System Interface."
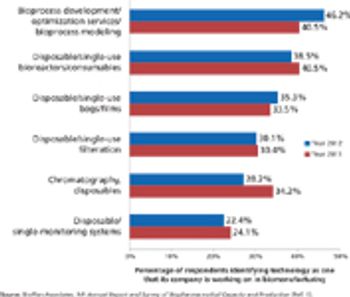
Industry wants more innovation, but can suppliers meet customers' needs?

This article contains online-exclusive supplemental material for Malik's article entitled, "Allogenic Versus Autologous Stem-Cell Therapy."

Collaborative R&D models coincide with new ways to fund translational research.

Unnecessary analytical testing can lead to unnecessary costs.

Although FDA cannot do anything to stop drugs from being discontinued, it can do something about supply and quality problems that lead to shortages.
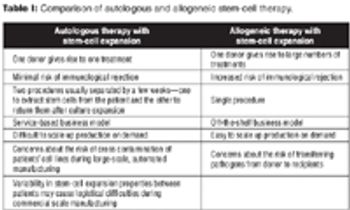
The author discusses potential manufacturing costs & challenges of allogeneic & autologous stem-cell therapy.
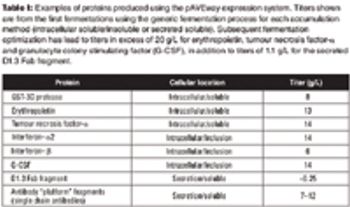
Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.
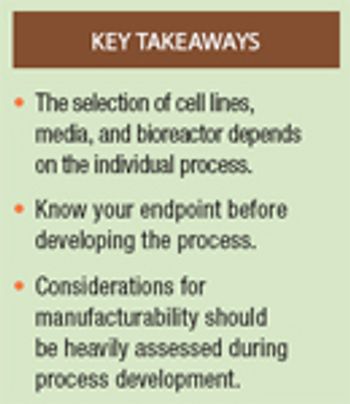
Selection of the right cell line, culture medium, and bioreactor conditions is key to setting up the upstream portion of the biopharmaceutical manufacturing process.
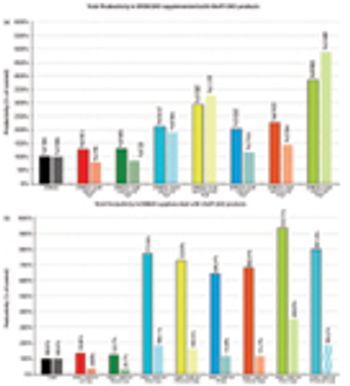
Cell-line specific complex media supplements combine chemically defined media additives into a single supplement.

Multiple initiatives are moving forward to maintain US leadership in biopharm R&D.
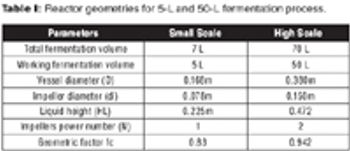
The authors present scale-up from a 5-L fermentor to a 50-L pilot-scale using the criterion of constant power consumption per unit liquid volume.
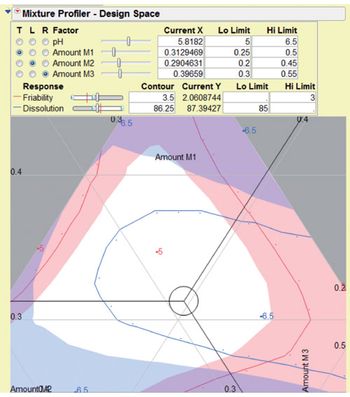
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.