
The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.
A controversial naming convention attempts to explain important distinctions between biologic drugs and their biosimilar counterparts.
Manufacturing for originator molecules is restricted by regulations, but drug makers can exploit newer technologies for the manufacture of biosimilars.

Drug type, potential sales, and ownership factor in the race to get drugs to market.

Siegfried Schmitt, PhD, Principal Consultant at PAREXEL, discusses how to mitigate risk in a global regulatory environment.
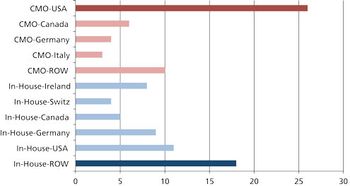
Moving global manufacturing operations may be more complicated than it appears.

As the November 2017 deadline nears, a surprising number of companies still don’t have a serialization plan in place. New programs aim to get them compliant in time.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall laboratory efficiency.

Communication and taking the time to develop the process are key to successful transfer and scale up of biologics
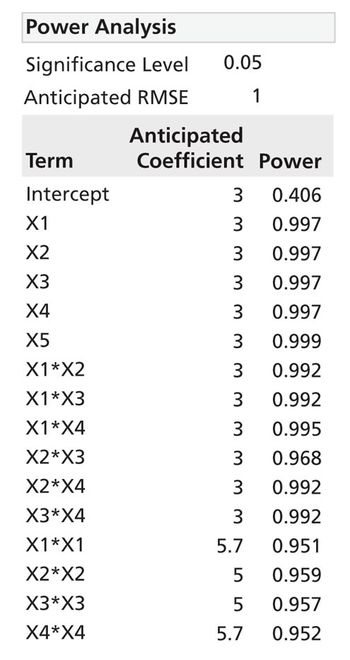
Process characterization and model building are essential skills and are required for modern drug development.

Johnson & Johnson Supply Chain (JJSC) and the distributor AmerisourceBergen launched a four-week pilot program to test GS1’s EPCIS standards and to see how effectively data could be transferred between the two partners.

The author discusses the results from TraceLink and Actionable Research's Global Drug Supply, Safety and Traceability Report.

Click the title above to open the BioPharm International March 2017 issue in an interactive PDF format.
Process controls get some upgrades to better reflect real-time conditions.