
Accelerated testing and production create challenges in documenting product quality.

Accelerated testing and production create challenges in documenting product quality.

China's regulatory and compliance environment is set to change as the government declares a crackdown on bribery scandals.
USP evaluates quality attributes for synthetic peptides.

UPLC System for Nano- to Microscale Separations
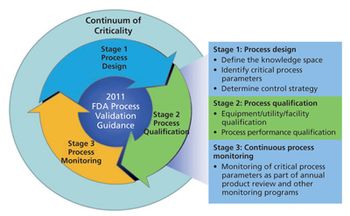
This series presents a practical roadmap in three parts that applies scientific knowledge, risk analysis, experimental data, and process monitoring throughout the three stages of the process validation lifecycle. In Parts I and II, risk analysis and process characterization studies were used to assign criticality risk levels to critical quality attributes and critical process parameters, and the concept of a continuum of criticality was established. In Part III, the author applies the continuum of criticality to develop the process control strategy and move through Stages 2 and 3 of the new process validation lifecycle.

Researchers are using current understanding of the lyophilization process to predict performance on many levels during both process development and manufacturing.

ALpHA G Capsule Filter for Single-Use Systems
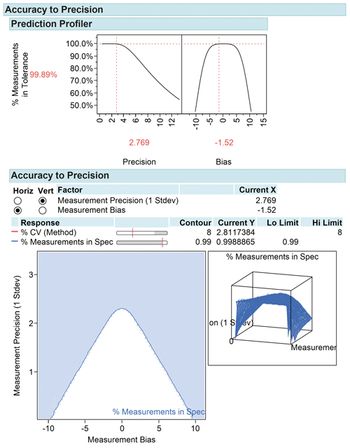
Design of experiment is a powerful development tool for method characterization and method validation.

Manufacturers are taking measures to comply with new package safety rules.
The targeted delivery of cytotoxic drugs using antibody drug conjugates would not be possible without effective linkers to connect and then release the key chemical and biological materials.

Changes in company ownership shake up the CMO industry.
Stain-Free Chromatography Workflow

Conference programming from PDA and BioPharm International expand educational opportunities at Interphex 2014.
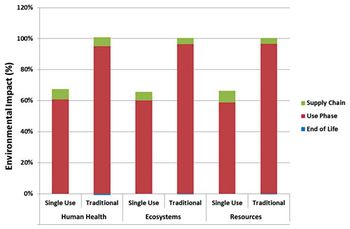
An environmental study of single-use process technology for biopharmaceutical manufacturing offers a comprehensive examination of environmental impacts across the full process train using lifecycle assessment.

A novel approach to sterile drug product manufacturing that uses a single-use assembly in a multi-product final filling suite with isolator technology offers benefits of efficiency and flexibility.

Click the title above to open the BioPharm International March 2014 issue in an interactive PDF format.