
Pricing pressures, investment volatility, and government disfunction greet Biopharma in 2019.

Pricing pressures, investment volatility, and government disfunction greet Biopharma in 2019.
For rapid scale-up of biomanufacturing under expedited review status, facility design must better integrate product development and manufacturing lifecycle activities.

Compensation and professional challenges are key pieces to solving the biopharma employee satisfaction puzzle.

Bio/pharma companies are successfully launching novel therapies; however, the industry still needs to work on manufacturing innovation.
Kinetic models can be used to study aggregation and fragmentation to help ensure stability.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

Automation in cell-line development and cell culture is leading to more consistent quality while improving efficiency, and, ultimately, speed to market.

FDA plans to support initiatives to ensure that all medicines are safe, effective, and of high quality.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.
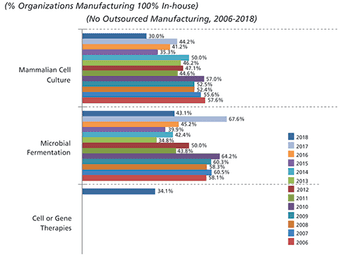
Outsourcing of manufacturing activities is expected to increase in 2019.

A key technology that can help achieve a continuous production flow is single-pass tangential flow filtration.

Click the title above to open the BioPharm International January 2019 issue in an interactive PDF format.