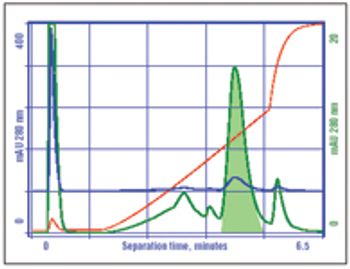
Manufacturing challenges surround the use of IgM monoclonal antibodies, but these can be overcome with current technology.

Manufacturing challenges surround the use of IgM monoclonal antibodies, but these can be overcome with current technology.
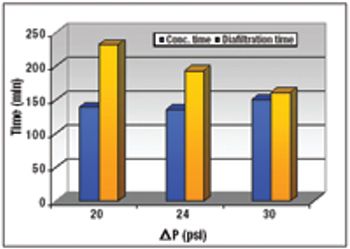
An alternative approach to traditional Protein A schemes is comparable in overall efficiency, product recovery, and quality.
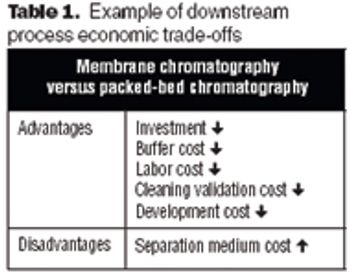
The future of therapeutic MAbs lies in the development of economically feasible downstream processes.
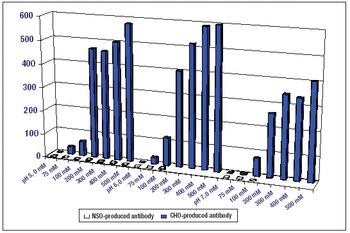
If certain engineering challenges can be addressed, precipitation may prove to be a valuable tool for antibody purification.

When platform processes are applied to fusion molecules, innovation and flexibility are needed.