
Subjective visual evaluation of freeze-dried products can be quantified through mechanical methods of characterizing the properties these materials.

Subjective visual evaluation of freeze-dried products can be quantified through mechanical methods of characterizing the properties these materials.

BioPharm International sat down with Kevin Isett, PhD, co-founder and CEO of Avitide, to find out why he thinks the company’s tailored approach to purification resins will change the face of biopharmaceutical separation.

Use of a subspace model is a viable method to characterize process space variables and optimize process performance.

The authors evaluate the SoloVPE technique as a replacement for nitrogen-based protein determination.

FDA confirmed quality focus while Congress moved to bolster biomedical innovation.

CMO industry consolidation may be frustrated by a dearth of attractive assets.

Expectations are high for rapid testing methods, but demonstration of comparability proves challenging.
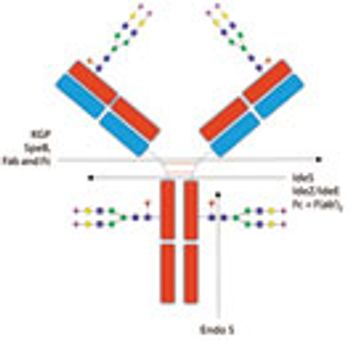
Advances in glycan analysis are enhancing biologics development and quality control processes.

Biopharma employees in different market segments note subtle differences in job satisfaction.

Finalizing GMP requirements and quality standards for the development, manufacture, and clinical testing of ATMPs in the EU is proving to be a complex task.

Limited career and salary growth complicate a somewhat positive employment picture.

Click the title above to open the BioPharm International December 2015 issue in an interactive PDF format.