A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services

A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.
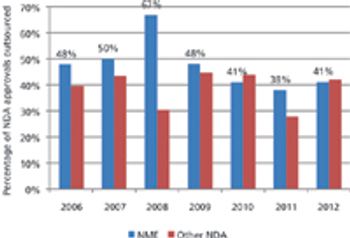
Approvals of new drugs are on an upward swing, but only a few CMOs are benefiting.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.
An integrated focus on product design, development, operation, and control.

The Taiwanese government engages in regulatory science in a move to boost its pharmaceutical sector.


Ranbaxy's $500 million settlement for producing adulterated drugs and fradulent data provides a cautionary tale for patients, FDA, and drug manufacturers.

Maintaining flexibility in biopharmaceutical manufacturing can deliver positive results.

A unique demographic and payer mix make ASEAN an increasingly attractive region.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

The authors provide common misconceptions and key concepts behind reliability engineering.

Click the title above to open the BioPharm International June 2013 issue in an interactive PDF format.