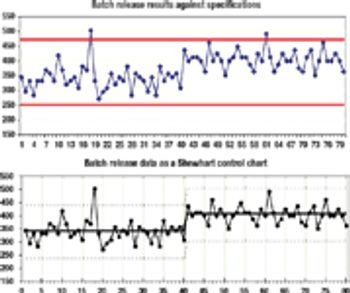
Improving your quality operations by using sound statistical principles.

Improving your quality operations by using sound statistical principles.
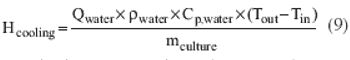
Process-modeling tools can ensure smooth tech transfer.

How to use hypothesis correctly, and understanding the difference between one-sample, two-sample, and z-test.
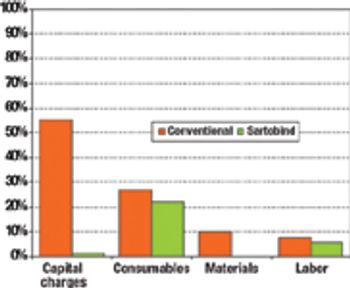
The current focus on cost-of-goods (COGS) models is underplaying the benefits of disposables technology in biopharmaceutical manufacturing. The best method for accounting for the benefits of reduced and delayed capital expenditures is through the use of NPV analysis.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.

The comparative research approach may be preferable to price controls in the guise of government negotiations for the Medicare drug benefit, coverage denials, and limits on access to new technologies.
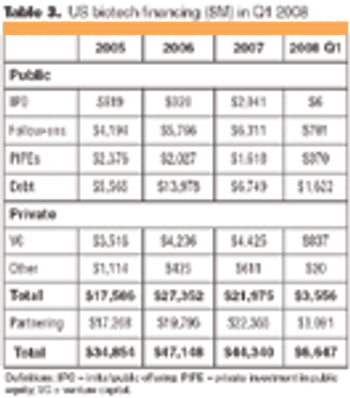
Following the market crisis of the first quarter of 2008, biotech IPOs and financing are down, but partnering continues, and mergers and acquisitions (M&As) remain hot.

A staged approach to limits should embrace future capabilities.

A stronger FDA will benefit both the industry and the public. And it now looks like we are starting down a path to build up that strength.

The industry needs to open up to validation failures.