
PDA’s New Technical Report for Biotech Cleaning Validation

PDA’s New Technical Report for Biotech Cleaning Validation

Executive management leadership is essential in the effective implementation of QbD.

Indian manufacturers are not now a threat to Western CMOs, but may be long term.
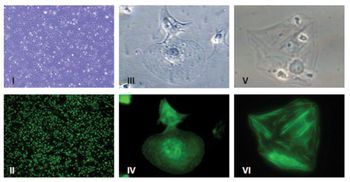
The authors describe an industrialized process for the manufacture of iPSC-derived human cardiomyocytes.

BioPharm International is the longest-running peer-reviewed publication dedicated to your work.

US Pharmacopeia develops and improves its class approach for ensuring quality biopharmaceuticals.
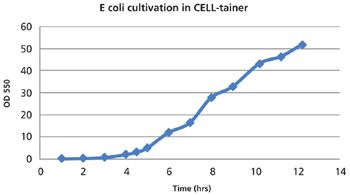
The authors discuss the use of single-use bioreactors.

The authors present an approach for testing statistical equivalence of two stability profiles.
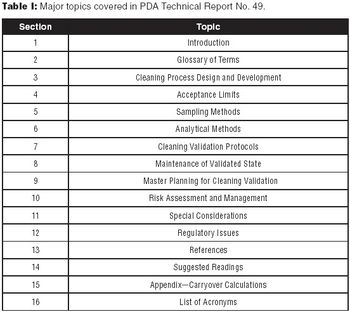
The authors encourage biotech manufacturers to consult PDA Technical Report No. 49 for a detailed perspective on current practices and issues in biotech cleaning validation.

As drug shortages make headlines, FDA tests the Sentinel safety system and its effect on healthcare.
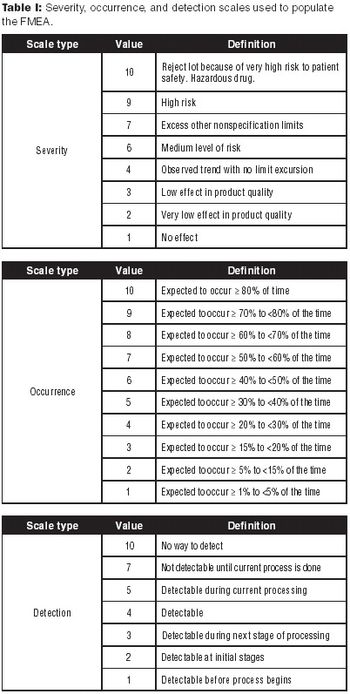
The authors review risk-assessment tools to evaluate product quality.