
Biopharma Navigates Stormy Seas

Biopharma Navigates Stormy Seas

How will implementing Quality by Design strategies affect your compliance status?

Demand for new vaccines and therapies in 2010 will be offset by concerns about drug prices and product safety.

A systematic classification system makes supplier quality management feasible, even if you are dealing with hundreds of suppliers worldwide.

The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.
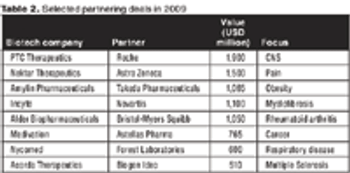
The companies that have survived the financial meltdown are well placed to adapt to the new environment that we now are entering.

Regulatory flexibility can make continuous improvement possible.
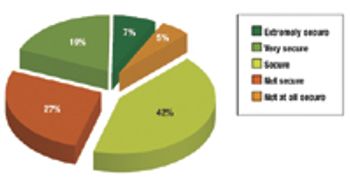
The year 2009 was marked by recession, industry megamergers, and venture-capital scarcity. How did biopharmaceutical professionals fare?

QbD has always embraced the notion that companies could make certain process changes without regulatory oversight.
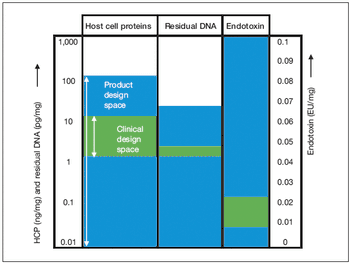
Key considerations for defining your overall control strategy.