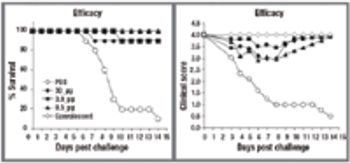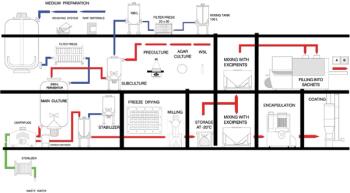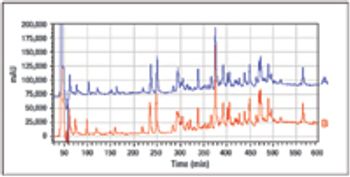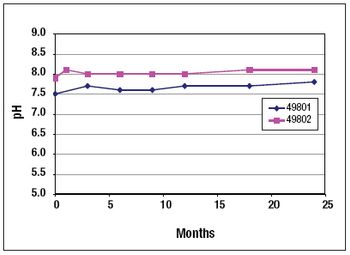
NeisVac-C is a polysaccharide-protein conjugate vaccine for use against Neisseria meningitidis serogroup C infection. The Phase 1 clinical formulation consisted of physiological saline, thimerosal, and aluminum hydroxide. The long-term stability data for both the PBS and saline formulations are presented in this article.