
Job Security in a Changing BioPharma Environment

Job Security in a Changing BioPharma Environment

A UC Berkeley survey provides insight into biopharma's risk concerns and strategies.
The author examines the use of closures for products intended for injection.

Preparation of biological samples for chromatographic analyses.

The South Korean market is characterized by its aging population and an affluent population. Growth potential is limited as it has evolved to a developed market and industry players expect it to have established regulations.

Keeping tabs on crucial medicines should be part of consumers' and manufacturers' emergency-preparedness plans.

White House and Congress likely to struggle over funding for bio/pharmaceutical regulation.

Can postapproval FDA filings immunize pharma companies from patent lawsuits?

An introduction to a new series on manufacturing within global markets.

Recent research examines the sequence of events underlying cellular reprogramming, which may aid in better control of the production of induced pluripotent stem cells.

NIBRT's Pauline Rudd on what to expect when performing glycan analysis.

Results from the 2012 employment survey.
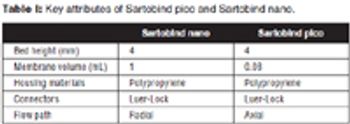
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.