
After many starts and stops, hype and disappointment, foreign protein expression in plants is now routine and biopharmaceuticals produced in green plants will soon be with us.

After many starts and stops, hype and disappointment, foreign protein expression in plants is now routine and biopharmaceuticals produced in green plants will soon be with us.

Chitosan and alginate hydrogels are high-molecular-weight carbohydrate polymers used in an extensive array of pharmaceutical applications. While many applications depend upon the ionized state of the polymer, other factors play an important role in the pharmacological activity of complexes that include these agents.
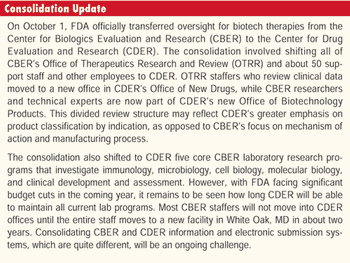
One goal of FDA's initiative to overhaul how it ensures high-quality pharmaceutical production is to apply more oversight and stricter rules to higher-risk products and processes (Inside Washington, October 2003).

UK science expertise, backed by increased government funding, has led to a thriving bioscience industry, distinguished by the energy and creativity of its entrepreneurs." These words are not mine but those of Lord David Sainsbury, the British government's science and innovation minister.

Biopharmaceutical products in storage change as they age, but they are considered to be stable as long as their characteristics remain within the manufacturer's specifications. The number of days that the product remains stable at the recommended storage conditions is referred to as the shelf life.

In a tale of two markets, shares in biopharm companies bounce back.

As FDA tries to bring pharmaceutical manufacturing into the 21st century, changes are likely to favor companies with greater manufacturing expertise and the means to invest in new technoglogies.