
Pfizer Tackles Lean and Six Sigma

Pfizer Tackles Lean and Six Sigma

A better method for trend analysis than CUSUM and control charts.
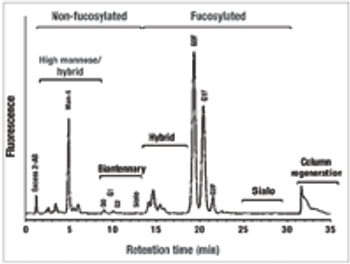
Select the best approach to determine critical quality attributes.

Perhaps the best way to regulate drugs is to regulate them not conservatively or liberally, but effectively.
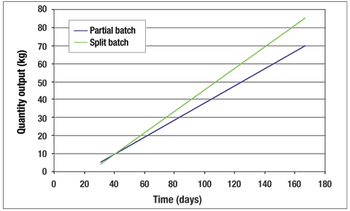
A case study in capturing indirect costs and benefits.

Enabling site-wide process efficiency.

The Bilski and Classen decisions can render numerous in vitro diagnostic claims unpatentable.

Contract research, development, and manufacturing organizations betting on a comeback in venture capital financing will have a long wait.

Pressures to reduce healthcare spending generates proposals to spur competition, cut costs.

Companies often wait for a critical mass before adopting new technologies. But if no one takes the risk, critical mass will never be reached.