
Wyeth today relies on biotech product revenue to drive approximately 25% of its revenues.

Wyeth today relies on biotech product revenue to drive approximately 25% of its revenues.

Understand your company's requirements, define responsibilities,and manage your team effectively.

Is it possible to reconcile phage therapy, which is inherently variable, with requirements for tight product characterization and control?

FDA did not gain any real teeth for regulating unsafe and ineffective products until a national health disaster in 1937 roused a public outcry.

When former Federal Reserve chairman Alan Greenspan's term in office expired on January 31, 2006, many people said, "There goes a legend." Maybe the better expression was, "Here comes the spin."

In 2005, 10 biopharma- ceuticals gained marketing approval in the US or Europe, although only five of them were genuinely new molecular entities.

Congress is not considering legislation that would expand FDA authority to regulate biologics.
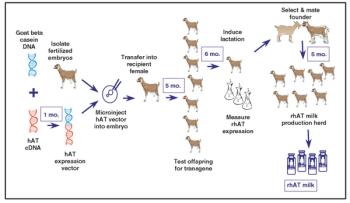
Capital investments in production plants represent a significant portion of the cost of new recombinant drugs
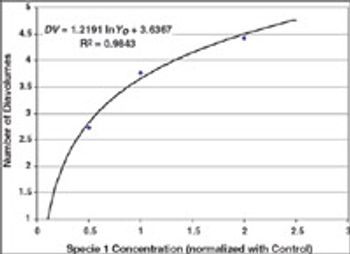
Case studies were run to test Process Analytical Technology applications for protein refolding, diafiltration, and cation exchange chromatography. It is shown that it is feasible to design control schemes that rely on measurement of product quality attributes and thereby enable real-time decisions.