
Robust Strategies to Optimize Processes

Robust Strategies to Optimize Processes
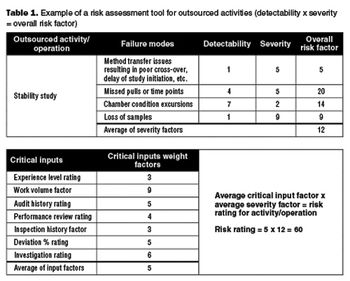
Apply risk management principles to monitor outsourced activities.

After a bright start to the year, some of biotech's blue chip companies have seen their early gains turn into losses.

How much to spend on early development, whether to use your CMO's proprietary cell line, and other outsourcing advice.

Identify the best experimentation methods for the data you need.
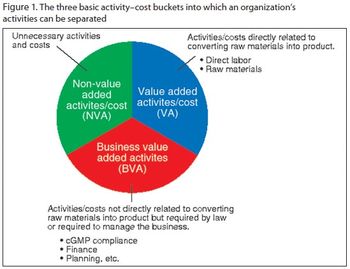
By identifying and eliminating non-value-added activities, drug manufacturers can avoid falling into the same cost-traps in the future.


A single standard should apply to all comparability exercises for biologics, be they for biosimilars or manufacturing changes.

USP is advancing efforts to develop a guidance for evaluating bioassays.
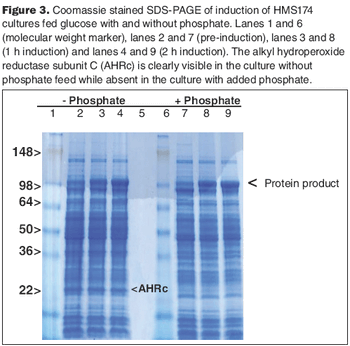
The HMS174 strain, in the absence or presence of excess phosphate, can metabolize acetate efficiently.

Twelve lessons of what to do and what not to do to avoid quality problems.

More information may be available on drug approvals, prices, and research to expand public understanding of regulatory policies.