Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.

Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.

Material compatibility, material sourcing, facility layout, and training are crucial aspects of a successful disposable fill-finish system.

The FlexPro 50, from Groninger, is a modular filling and closing system designed to process vials, cartridges, and syringes, as well as vials in bulk and trays.

The Optima AUC from Beckman Coulter Life Sciences is an ultracentrifuge that determines molecular weight, size shape, and polydispersity.
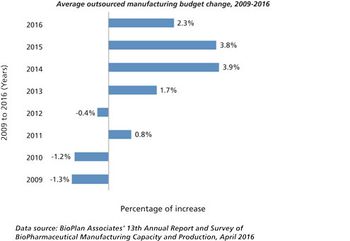
Growth may be slowing, but outsourcing activity remains healthy.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.

Policies for patient access to life-saving therapies must keep pace with biomedical innovation.
Early successes drive the need to overcome safety issues, increase efficacy, and address manufacturing challenges.

Developers of CAR-T cell therapies with products showing successful early-stage clinical results are currently seeking manufacturing capacity that will enable the production of the larger quantities of material needed for Phase III trials and eventual commercialization.

Two main safety issues have been identified in the early-phase clinical trials conducted to date for chimeric antigen receptor (CAR)-T cell therapies.

Cell therapy companies are attracting interest from investors, and drug companies are seeking partnerships and acquistions to accelerate development.
Testing product and process intermediates alone is helpful, but does not provide a complete solution to viral safety. This article proposes integrated solutions for systemic and proactive viral risk mitigation.

Click the title above to open the BioPharm International May 2016 issue in an interactive PDF format.

The campaign against opioid abuse opens door to more innovative therapies.
The authors provide their perspectives on shipping validation.