
The challenge will be to design a system that is flexible, yet appropriate, for the broad range of biological products and the varying quality control capabilities of different manufacturers.

The challenge will be to design a system that is flexible, yet appropriate, for the broad range of biological products and the varying quality control capabilities of different manufacturers.

Process monitoring ensures that the process performs within the defined acceptable variability that served as the basis for the filed design space.

Financial pressure to show short-term results also affects the pharmaceutical industry, from Big Pharma to small biotech.
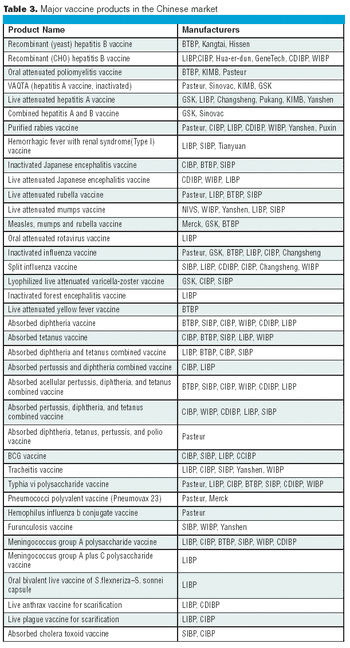
The Chinese vaccine market competition is now transferring from the former price-competition model to a technology- competition model.
Frequently asked questions on implementing and using single-use technologies

This 1,230-page tome is a must-have encyclopedia for any person or organization planning to interact with the biopharmaceutical market in China. It contains 23 well-written chapters and five appendices written by individuals who are experts in the areas they address. Some of these experts are not well known in the US; thus the book also provides an excellent introduction to people whose knowledge and opinions are important when considering biopharmaceuticals in China. Many chapters were translated from Chinese and this is the first time that their information has been provided to the West.

Virtually every corner of the United States (not to mention the rest of the world) seeks to build a powerful biotech presence (see www.bio.org/local/). Since the dawn of biotechnology in the mid 1970s, private venture capital, major corporations, and state and federal governments have poured hundreds of billions of dollars into the industry. Results have been mixed, in terms of benefits to local economies and products that reached the marketplace.

Scale-up issues leading to long development times and deviations in the commercial facility is a critical challenge.

Although contaminants and other parameters may be main causes of filter breakdown, some nanofilters still remove viruses at high Log Reduction Value (LRV).