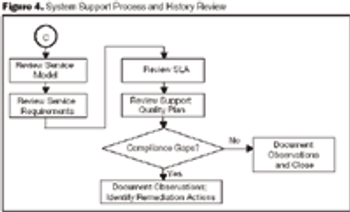
Organizations can mitigate the risk of failure during regulatory inspections by identifying and correcting deficiencies before an inspection occurs.

Organizations can mitigate the risk of failure during regulatory inspections by identifying and correcting deficiencies before an inspection occurs.

Disposable products and systems have come a long way since they first entered the small-lab market in the 1970s. Today they are available for practically every aspect of biopharmaceutical manufacturing. Disposable systems are used for filtration, clarification, purification, and separation applications used in the production of vaccines, monoclonal antibodies, and other therapies. As the use of disposable systems grows, the concept of a completely disposable manufacturing process is becoming a reality.

In most production environments, it is critical to be able to associate historical environmental data with process data.

One of the critical early tasks of the documentation team is coordinating plans and requirements with the start-up team.

The growing alarm over harmful side effects from a number of popular prescription drugs is affecting a range of issues of critical importance for pharmaceutical and biotech manufacturers. Safety concerns may slow down efforts to expand drug importation from foreign nations. The National Institutes of Health (NIH) has halted important clinical trials due to fear that the painkillers under study increase risk for cardiovascular events.

The contract manufacturing sector has not enjoyed the success of preclinical and clinical businesses.

Speed of response, small-scale manufacturing and process flexibility will become increasingly important.
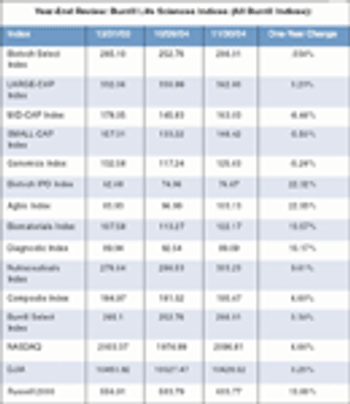
I wrote in the December 2005 issue of Streetalk that no matter who won the election between George Bush and John Kerry, the result would have been good news to the biopharm industry - if for different reasons. I said that with President Bush back in office for a second term, industry red tape would be diminished and the life sciences sector would shift into higher gear.

In the first part of this feature (Jan. 2005) we discussed the technical background and the role that FDA, US Department of Agriculture (USDA), and Environmental Protection Agency (EPA) play in setting the rules for accepting plant-made biopharmaceuticals (PMBs). We now continue by discussing how producers will be able to take products to market.

Unfortunately, once circuits are commissioned and validated, optimizing - or even adjusting - them is difficult and rarely done because of the time and effort required for revalidation.
The bulk of a biopharmaceutical processing unit can be assembled with off-the-shelf components. However, special fabrications — especially fluid components — enable fabricators and manufacturers to meet critical construction deadlines and move projects forward with minimal or no delays.