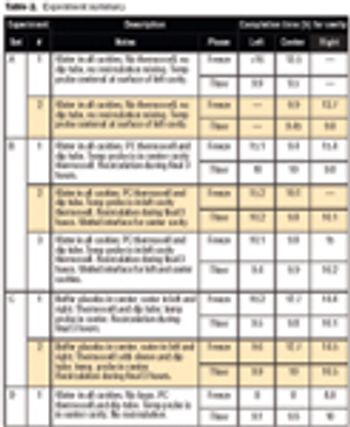
A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.

A solution for the problems of a "bag in a can" system would be a fully jacketed and insulated container, similar to a traditional freeze tank.
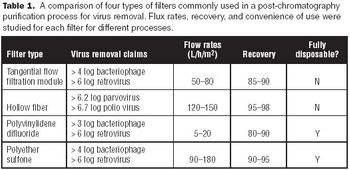
One of the challenges of adopting single-use technology is that not all cell lines are compatible with disposable bioreactors.
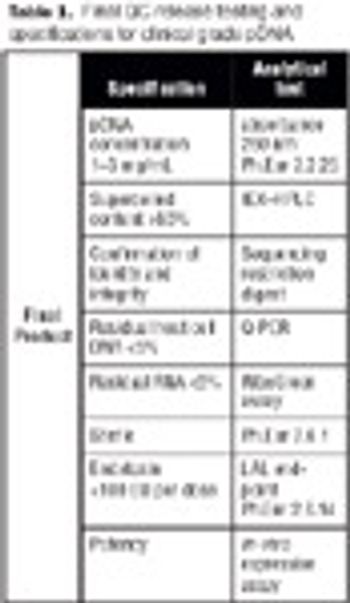
Disposables are increasingly being used in the manufacture of biopharmaceuticals. This article describes the design of a fully disposable process for the cGMP manufacture of clinical trial grade plasmid DNA. It addresses the rationale for implementing such a process with respect to the manufacture of patient-specific plasmid DNA vaccines for the treatment of leukemia. The process incorporates a number of disposable technologies, which are simple to use and thus reduce the need for investment in expensive equipment and cleaning validation.
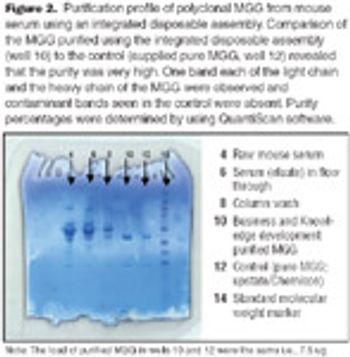
Comparison of the integrated assembly purified MGG to the control revealed that the purity if the MGG was very high.
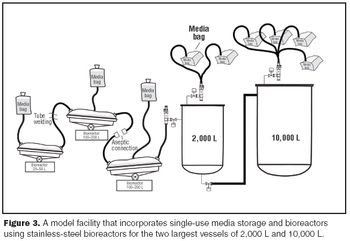
Single-use systems reduce maintenance and capital expense by eliminating expensive vessels, valves, and sanitary piping assemblies.