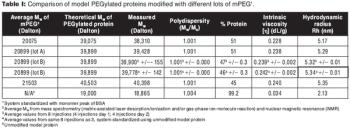
Reliably detecting low amounts of high molecular weight impurities during process development and characterization of biopharmaceutical products.

Reliably detecting low amounts of high molecular weight impurities during process development and characterization of biopharmaceutical products.
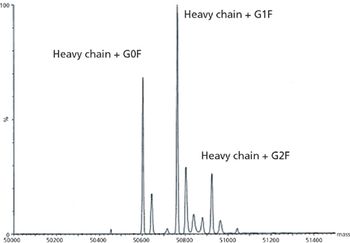
The author describes techniques that can be used to provide the analytical data required by ICH Q6B for characterization of monoclonal antibodies.
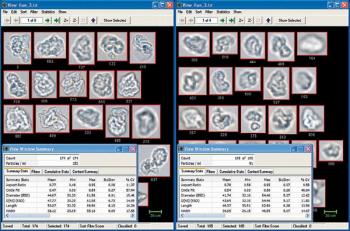
Overcoming limitations of volumetric techniques and detecting transparent particles.

Advances in Protein Characterization