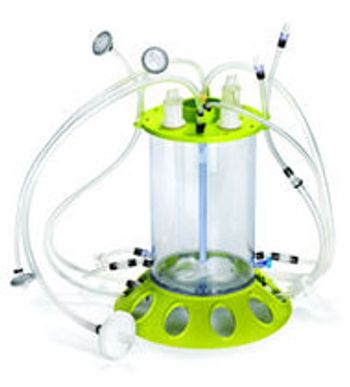
Review the process-design space and scalability of a single-use, stirred-tank bioreactor.

Review the process-design space and scalability of a single-use, stirred-tank bioreactor.

Jerold Martin considers the types of tubing available to the industry and how to make an informed selection.

Reduced carryover risk and close attention to particulate control result in greater patient safety through the use of single-use systems.

BioPlan's Annual Report shows continued growth in the use of single-use technology.
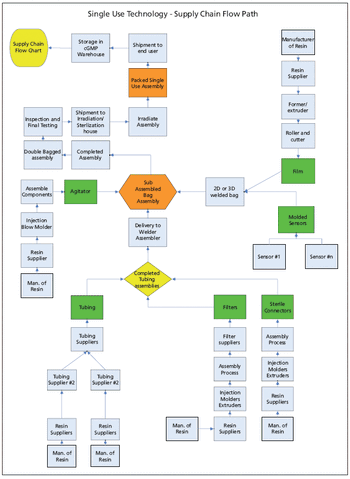
The authors suggest techniques for mitigating risk and securing the supply chain for single-use components used in biopharmaceutical manufacturing.
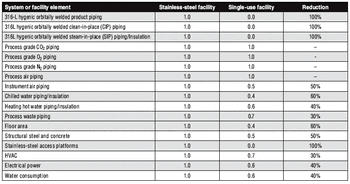
The move to single-use manufacturing has prompted a paradigm shift in facility design.