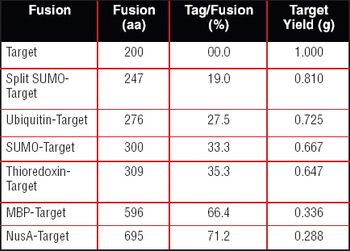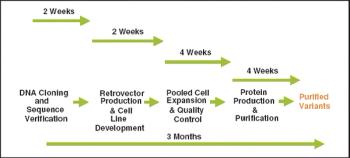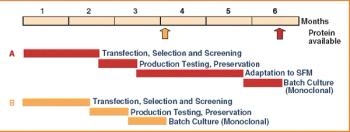
Over the last three decades, numerous protein expression systems have been developed with various quality requirements on large and small scales. Huge steps have been made in large-scale protein production in mammalian systems while the small-scale mammalian systems are expensive and inflexible. Thus, small-scale production is done in simpler expression systems, sometimes sacrificing the quality of the proteins. However, relief is on the way.