
Three case studies illustrate some analytical methods important for stability testing.

Three case studies illustrate some analytical methods important for stability testing.
Focusing on niche and specialty service offerings gives contract biomanufacturing organizations an opportunity to differentiate in a crowded market.

imagewerksBiophysical binding studies utilizing surface plasmon resonance, biolayer interferometry, isothermal titration calorimetry, or related techniques are ce

Sorendls/Getty ImagesTo maintain a state of control and comply with regulatory authorities, many pharmaceutical, biotech, and medical-device companies have adopted continued pro

Legislation to streamline drug development may get tangled up in user fee negotiations and drug pricing battles.
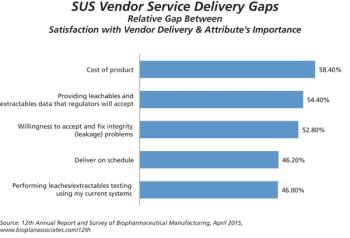
Suppliers indicate prices for single-use equipment are likely to increase.
Meissner’s FlexGro single-use biocontainer assemblies feature the TepoFlex polyethylene (PE) multi-layer film and are delivered presterilized for immediate use.
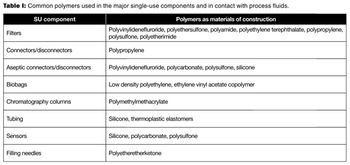
The author discusses the current best practices in technical qualification of single-use systems.

FDA’s proposed guidance for quality metrics raises questions about quantifying the tangibles and intangibles of quality culture.

Ensuring data integrity involves effort on an individual and global basis.
New single-use technologies and other filtration systems are beginning to address cost, throughput, and manufacturing footprint demands.

Click the title above to open the BioPharm International September 2015 issue in an interactive PDF format.