
Essential Steps to Ensure Accurate Filter Scale-up

Essential Steps to Ensure Accurate Filter Scale-up

If risk assessments only identify "the usual suspects," the process will not add much value.
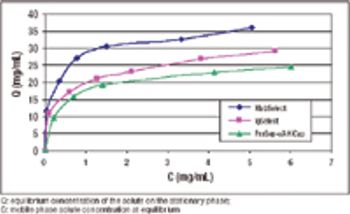
A stable alternative to Protein A chromatography.
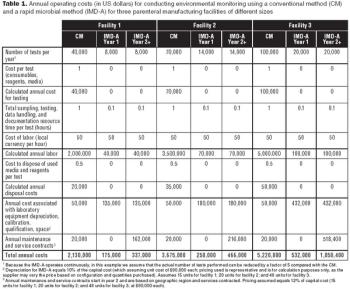
A case study implementing rapid microbiological methods.

Recent patent rulings raise significant patentability questions for DNA sequence inventions.
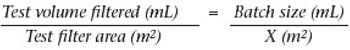
Why staining is crucial in flow decay studies.

The FDA is encouraging manufacturers to invest in research and development for new vaccines and therapeutics to combat third-world diseases.

ICH Q9 encourages companies to apply the concept of quality risk management. Easier said than done.
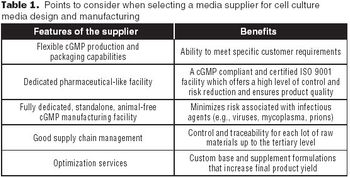
To select the right partner for media design and optimization services, several key factors must be considered.