
Biotechnology players are emerging in countries that historically have not been substantially active in this industry.

Biotechnology players are emerging in countries that historically have not been substantially active in this industry.

FDA's regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures provides industry with the requirements that allow electronic records and signatures in computerized systems in place of paper records and handwritten signatures.1

There are six mechanisms for bringing a veterinary biologic into use when circumstances demand a rapid response and full licensure will take too long.

Introduction - Gene therapy is a medical intervention based on the modification of the genetic material of living cells. Currently, gene therapy is restricted in application to somatic cells.

Government agencies examine the need for more regulation of ?bio-pharming,? biosecurity, and BSE.
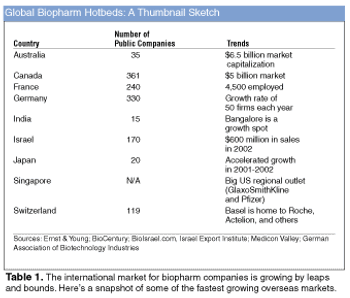
The global market for biopharmaceuticals points to a healthy future for industry.

In October 2003, Shenzhen SiBiono GeneTech made history by becoming the first company approved to market a gene therapy medication. China's State Food and Drug Administration (SFDA) approved Gendicine for treatment of head and neck squamous cell carcinoma (HNSCC). SiBiono believes that continued clinical trials will prove Gendicine to be effective as a wide-spectrum anticancer agent.

As biotechnology organizations have successfully launched new products, the challenges of producing adequate quantities have grown. Many companies are now dealing with multiproduct manufacturing facilities and pushing the limits of their capabilities. One result of this complexity is a loss of production capacity due to inefficiencies.

Plan ahead to make the most of every opportunity at this year?s BIO convention in San Francisco.