
The Quality Product Steward Model

The Quality Product Steward Model
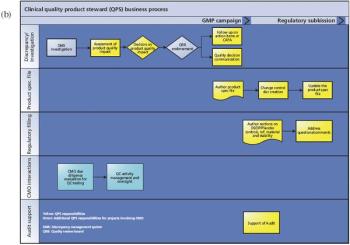
A successful QPS acts as a single point of contact for consistent product quality oversight.
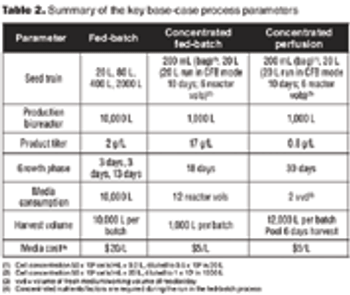
Comparing the economic feasibility of a typical glycosylated protein.

Those at the top must walk the walk of uncompromising commitment to compliance

Biotech companies will have a much better shot at success with a project management plan and team in place from the very outset

Although some aspects of single-use components can be standardized, it is unlikely that any materials or design features will become a commodity

FDA prepares for top-level changes while promoting transparency and product safety

An approach to reduce batch time, increase productivity, and decrease costs.

Capable of great works, pharma too often yields to the lesser angels of its nature