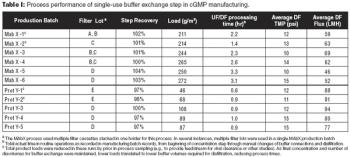
This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.

This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.

This article describes best practices for implementing a single-use process train at a bioproduction facility.
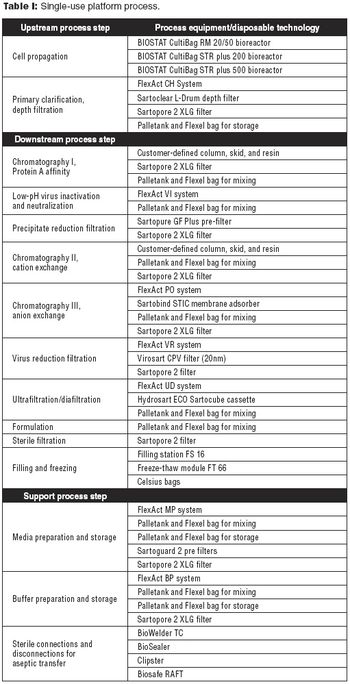
The combination of single-use platform technology with modular facility construction is a template for flexible manufacturing.

A brief case study of a facility-fit analysis provides insight into how to adjust capacity when moving from clinical-to commercial-scale production.

The authors explain why Catalent decided to transition from stainless steel to single-use systems.

GE Healthcare Life Sciences' ReadyToProcess platform aims to streamline bioprocessing.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.