For pandemic vaccine processing, single-use filter cartridges and membrane chromatography technologies could offer significant time- and cost-reduction advantages.

For pandemic vaccine processing, single-use filter cartridges and membrane chromatography technologies could offer significant time- and cost-reduction advantages.
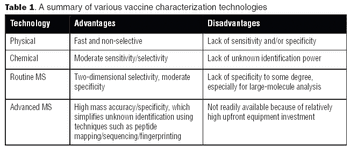
With the advent of high-resolution mass spectrometers and highly sensitive MS instruments, vaccine characterization has entered a new phase.

In animal studies, we have demonstrated that the dose of an injected H5N1 vaccine candidate can be significantly reduced by using a skin patch containing E. coli heat-labile enterotoxin (LT) applied over the injection site. LT-activated epidermal Langerhans cells migrate to the nearby draining lymph node and enhance the immune response to the injected antigen. A dry patch formulation has been optimized as a dose sparing strategy for pandemic flu and other vaccines. Iomai Corporation has developed a proprietary stabilizing formulation for the patch that allows use and storage at ambient temperature. The patch withstands temperature extremes during shipment, and is suitable for stockpiling.
For many cell-based vaccines, the precursor monocytes or CD34+ cells are cultured with cytokines to obtain dendritic cells, which are very potent antigen-presenting cells (APCs).

The recent growth in the vaccine market has led to renewed interest in using adherent human cell lines for vaccine production. Traditionally, small-scale adherent cell line production has been carried out in roller bottles or T-flasks. Over the past few years, however, a number of companies have found multi-tray disposable bioreactors an effective method for producing high-quality drug products using adherent cells. These disposable, expandable systems have also facilitated scale up from laboratory to clinical-scale.

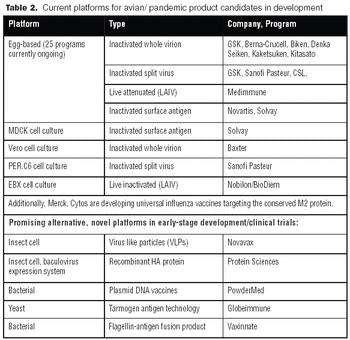
Vaccines against strains originating from avian flu may achieve poor yields in egg-based systems. Consequently, both public and private interest in alternative systems is high.

Any endpoint considered appropriate to support approval, whether a surrogate or a clinical endpoint, must be supported by substantial evidence of effectiveness.
Like the egg-based vaccine production process, producing a vaccine under cGMP conditions using mammalian cells can be a lengthy process, taking a minimum of six to 12 months.
Adjuvant-caused vaccine reactions are one of the most important barriers to better acceptance of routine prophylactic vaccination.