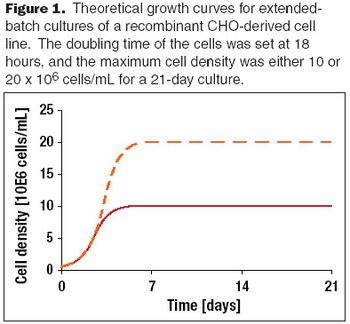
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.

A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.

With a variety of recombinant, animal-free, defined protein supplements such as growth factors, transferrin, and albumin entering the market, the biopharmaceutical industry now has innovative and safer alternatives to serum and other animal-derived supplements.
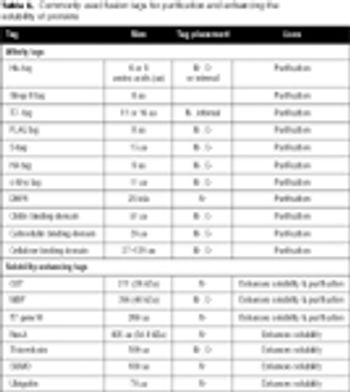
Gene fusion tags can improve the yield and solubility of many recombinant proteins. This article discusses the most popular fusion tags and the proteases used to remove them, with special reference to recently introduced technologies.
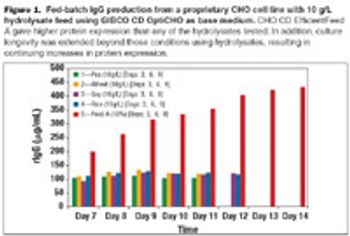
Using chemically defined feeds with CHO cell lines not only eliminates the variability associated with using plant hydrolysates, but could also improve the productivity of biopharmaceutical protein manufacture and help move therapeutic proteins into clinical trials more rapidly.
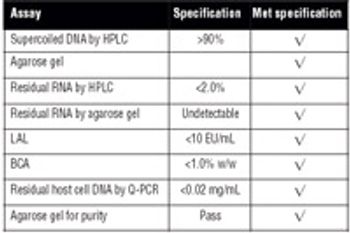
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.