Networks are part of the compliance picture. Recent FDA warning letters show the agency considers network monitoring and qualification a necessary part of maintaining the security and integrity of electronic records.

Networks are part of the compliance picture. Recent FDA warning letters show the agency considers network monitoring and qualification a necessary part of maintaining the security and integrity of electronic records.

The FDA rule on electronic signatures and electronic records was issued in 1997, but the details of implementation are still being debated. The 2003 FDA guidance redefines the scope of 21 CFR Part 11. Understanding which records now fall under the scope of the rule can help you begin implementing your compliance plan.

The FDA?s risk-based approach to pharmaceutical cGMPs applies to 21 CFR Part 11 enforcement as well. Understanding different methodologies for assessing and managing risk will help you develop and begin to implement a compliance plan.

Protecting the integrity of data is a challenge of 21 CFR Part 11 compliance. Integrity requires records to be complete, intact, and maintained in their original context ? associated with the procedures which were used to create the data.
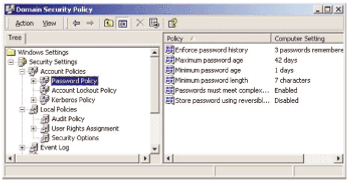
How can you be sure only authorized users are entering data into your system? Is your electronic signature yours alone? Are you sure operators can?t invalidate your data? Is your company in compliance with FDA data security regulations? The second article in our series on 21 CFR Part 11 will help you answer these questions.

Bringing different laboratory instruments into compliance takes planning. The key strengths and weaknesses of different levels of control and feedback for analytical instruments and data transfer systems are highlighted in this article.