
How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.

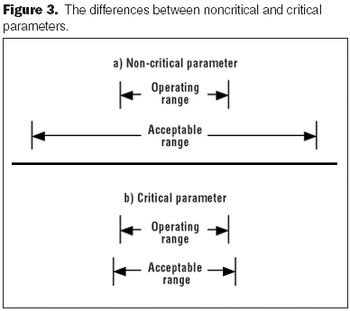
Developing a process validation strategy early in clinical development is critical for a successful validation program.

China's pharma industry represents 5% of the world total. By 2010, China is expected to become the world's fifth largest pharmaceutical market.

Eighty drugs will lose patent protection between 2007 and 2011.

Many companies acknowledged that Western regulatory standards are tougher than those in China.

More than $1 out of every $9 under professional management in the United States is involved in socially responsible investing.

The personalized medicine bandwagon is on a roll, offering a new model for calculating reimbursement of high-cost biotech therapies. Strategies for identifying patients who will respond to a certain therapy, as well as those most likely to suffer adverse events, promise to improve healthcare quality while eliminating waste and inappropriate spending. Interventions based on individual genetic characteristics may have limited sales, but support higher prices and less costly clinical research methods.