
Jon S. Kauffman, PhD
Advertisement
Jon S. Kauffman, PhD, is the director of method development & validation and biopharmaceutical services at Lancaster Laboratories
Articles by Jon S. Kauffman, PhD

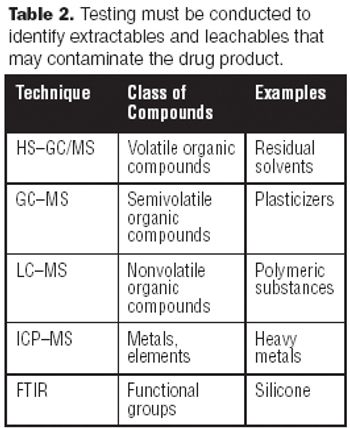
A tremendous amount of analytical testing is required to support a biopharmaceutical product from discovery, development, and clinical trials, through manufacturing and marketing. Numerous methods are used to fully characterize large molecules because of their complexity-characterizing them is significantly more difficult than it is for small molecules. Biopharmaceuticals are produced via living systems, i.e., E. coli, yeast, or mammalian cells, which require additional testing matrices.
Advertisement
Latest Updated Articles
 Analytical Strategies for Monitoring Residual Impurities
Analytical Strategies for Monitoring Residual ImpuritiesPublished: December 18th 2009 | Updated:
 Analytical Testing to Support Biopharmaceutical Products
Analytical Testing to Support Biopharmaceutical ProductsPublished: April 2nd 2007 | Updated:
Advertisement
Advertisement
Trending on BioPharm International
1
How CDMO Alliances Can Provide End-to-End Service that Reduces Drug Development Time and Costs
2
FDA Clears PharmaResearch IND for Nano-Based Cancer Drug PRD-101
3
First-in-Human Study Validates Safety of Next-Generation mRNA–LNP Platform
4
High-Dose Nusinersen Slows Neurodegeneration in SMA Patients, Study Shows
5
