
Webinar Date/Time: Wed, Oct 22, 2025 11:00 AM EDT

Webinar Date/Time: Wed, Oct 22, 2025 11:00 AM EDT

Webinar Date/Time: Wed, Sep 24, 2025 11:00 AM EDT

Tue, Jul 15, 2025 11:00 AM EDT

Webinar Date/Time: Thu, May 15, 2025 11:00 AM EDT

Webinar Date/Time: Thursday, December 5th, 2024 at 11:00 AM EST

Key strategies for accelerating development and manufacturing and development timelines to bring drugs to market faster and give developers a competitive advantage.

Webinar Date/Time: Wed, Mar 20, 2024 11:00 AM EDT

Webinar Date/Time: Thu, Feb 22, 2024 11:00 AM EST
Webinar Date/Time: Wed, Dec 13, 2023 11:00 AM EST

Custom Protein Production at the Highest Levels of Quality and Speed

WuXi biologics provides end-to-end biologics and vaccines solutions.
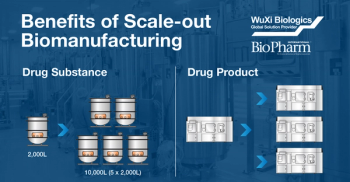
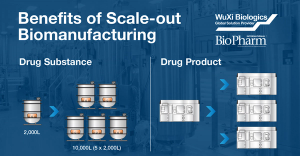
Changes in production scale required due to increasing product needs throughout clinical trials introduces risk to the product development timeline. Both the process and the product can be dramatically impacted from scaling-up to larger production volumes. Once in commercial manufacturing, sudden changes to product demand in either direction can have costly consequences if the scale of production chosen is not adequate to meet the changing material requirements. Adapting a “scale-out” instead of “scale-up” strategy for clinical and commercial production can greatly minimize these risks. This podcast will review the scale-out biomanufacturing approach and the significant benefits of this production paradigm.

WuXi biologics provides end-to-end biologics and vaccines solutions.



***Live: Thursday, Dec. 3, 2020 at 11am EST| 8am PST| 4pm GMT| 5pm CET*** Review preclinical strategies to improve drug development outcomes for biologic drugs, including innovative solutions and risk-based approach to expedite investigational new drug submissions, especially for pandemic response initiatives.. ***On demand available after final airing until Dec. 3, 2021***
Published: November 22nd 2021 | Updated:

Published: March 1st 2021 | Updated:

Published: June 18th 2025 | Updated:

Published: October 18th 2021 | Updated: