Analytical Method Lifecycle: A Roadmap for Biopharmaceutical Development
The authors propose a roadmap for analytical lifecycle development.
ABSTRACT
Analytical method lifecycle is a continuous process that improves and documents the understanding of the capabilities of each analytical method used throughout the clinical development of a new drug candidate. Of key importance, analytical lifecycle-related activities have to be appropriately staged in accordance with the regulatory requirements without neglecting the financial and time constraints incurred by each project. Currently, regulatory requirements for analytical methods are primarily directed at prerequisites for commercial manufacturing, the end point of the development process, without any description of requirements regarding the stepwise development leading to validation. This article proposes an analytical lifecycle roadmap that will stage the various steps involved in analytical method development while attempting to meet the expectations of the stakeholders involved in the management of project risk, development costs, and regulatory compliance.
Despite the growing quality-by-design (QbD) trends that promote accumulating characterization data from early clinical development, a number of biotech companies are constrained to demonstrate early positive clinical results within a shorter timeframe and with less money than before. Often, these companies have a business model to sell or license the product under clinical development to a larger pharmaceutical company for final development and marketing. The value of the product will then be determined by a number of factors including indication, product safety/efficacy data, and process development status.
Photo Credit: Getty, Design Pics/Bilderbuch
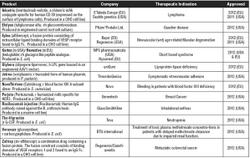
Biotech and pharmaceutical companies involved in the process development and clinical manufacturing of a new biologic drug candidate have to achieve the right balance between development goals and inherent project risk, cost, and regulatory compliance at the different stages of clinical development (see Table I). The development strategy should therefore rely on phase-dependent yet flexible business decisions that take into account all the elements of risk mitigation, cost balance, and compliance towards regulatory requirements, an approach that will of course affect the different aspects of analytical work. In this context, a lifecycle approach has the benefit of offering a long-term vision of the project associated with sustainable business decisions to the stakeholders involved.
Table I: Project overview in clinical development.
Analytical method lifecycle refers to the combined activities of analytical method development, improvement, qualification, validation, transfer, and maintenance related to GMP production. An integrated approach to analytical lifecycle must ensure that analytical methods evolve from initial development to commercial use in a manner that is best suited for their intended use at the various clinical stages on the way to commercialization. Building an analytical lifecycle roadmap that satisfies all requirements must be supported by strong technical expertise as well as sound business and regulatory knowledge.
REGULATORY FOUNDATIONS
At the moment, only analytical method validation is well regulated. Detailed information on analytical method validation can be found in ICH Q7A, ICH Q2, some FDA guidelines, US and EU compendia as well as technical guides issued by nonprofit groups such as the Parenteral Drug Association (PDA) (1). Other well-regulated aspects of drug development are linked to dossier submission requirements for clinical trials and although the specific requirements with respect to analytical methods are not well described, these documents have an impact on analytical method lifecycle. These documents include:
- Investigational new drug (IND) submission for Phase I and for Phase II/III in the US (2, 3)
- Investigational medicinal product dossier (IMPD) submission for clinical trials in Europe (4, 5)
- FDA/EMA's guidelines on process validation (6, 7)
- ICH M4Q on common technical document (CTD) module 3—quality section
- ICH Q5C on stability testing
- ICH Q6 on test procedures and acceptance criteria
- ICH Q8–11 on pharmaceutical development, risk management, and quality systems.
In Europe, the EMA guidelines on IMPD submissions state that "...for Phase I clinical trials, the suitability of the analytical methods used should be confirmed. The acceptance limits (e.g., acceptance limits for the determination of the content of impurities, where relevant) and the parameters (e.g., specificity, linearity, range, accuracy, precision, quantification, and detection limit, as appropriate) for performing validation of the analytical methods should be presented in a tabulated form." (4, 5) The analytical methods should be validated before Phase III studies, although it may be not appropriate to engage resources in formal ICH validation for Phase II submission with respect to the limited level of knowledge on product and process. Likewise, in the US, appropriate validation data should be provided for the analytical procedures for Phase II/III, although it must be confirmed for Phase I that the method is scientifically sound, suitable, and reliable for its intended purpose (2, 3).
Interestingly, both EMA and FDA guidance documents describe the requirement that the method, scientific intent, and performance be assessed at an early stage when the project is transferred from process development to GMP production. Moreover, these guidelines set the pace for initiating exploratory "prevalidation" work for setting ICH-compliant acceptance criteria used in validation. In other words, there should be something done at early clinical stage to confirm that the method is scientifically sound and of reliable method performance before formal ICH validation is done later in clinical stage.
PREPARING ANALYTICAL METHOD VALIDATION
Prevalidation, also known as qualification, ranging from initial performance assessment to method refinement and robustness assessment has to be smartly staged in the course of the project (8). Qualification, while not an official term employed in analytics-related regulatory guidelines, is often encountered as the equivalent term referring to analytical activities starting after the development of the method and ending with the assessment of method validation readiness (see Table II). Unfortunately, there is little information available in guidelines about regulatory expectations regarding qualification compared with validation. It is then part of the project sponsor's duty to establish its rationale for the analytical method lifecycle during clinical development.
Table II: Analytical method qualification factsheet.
Different types of analytical lifecycle activities can occur before formal method validation. These activities typically include the development of the analytical method per se, an initial performance assessment strongly encouraged for Phase I, robustness assessment as part of ICH Q2 prerequisites, and probably some method refinements in terms of improvement (e.g., for better performance) and optimization (e.g., for higher throughput). The scientific rationale of the method comes during development as a function of the target product profile, critical quality attributes, process outline, target method performance, prior knowledge, and scientific expertise.
STEPS IN ANALYTICAL METHOD QUALIFICATION
In contrast to analytical method validation where regulatory requirements are explicit, qualification requires the project sponsor to have a clearly defined policy in the absence of well-defined regulatory boundaries. Ideally, qualification starts with an initial method assessment for filing the IMP dossier for Phase I. This assessment can be done immediately after method development, keeping in mind ICH Q2 parameters, with the aim of providing authorities with first results on method performance and the setting of validation acceptance criteria for future ICH validation. Of course at this early stage, cost constraints can be an impetus for reducing the burden related to cGMP (e.g., in terms of quality assurance oversight), provided that confidence and reliability in data acquisition and management are ensured.
While not cited in ICH Q2, stability-indicating profile of methods used to demonstrate product stability should be addressed as part of the analytical method lifecycle in accordance to ICH Q5C on stability, at the latest during validation. Conditions known to affect product stability (that have been determined from prior preformulation development work, stress stability studies, and accelerated stability studies) are useful for showing stability-indicating properties of analytical methods. The whole project can always benefit from the confirmation that analytical tools are stability-indicating before initiating pivotal stability studies or preferentially earlier during method development and initial performance assessment. A good practice in sample selection is to include one batch of representative material as well as its degraded forms.
The next step in qualification can include method refinement and robustness assessment, preferentially performed during Phase II. Refinement typically includes finding the optimal way to run the test method in the laboratory, whereas robustness assessment allows identifying critical parameters affecting method performance. These complementary activities, however, do not supersede results from the initial performance assessment since non-inferiority criteria (at least equal to) are applied. Moreover, applying QbD principles at this stage (i.e., design of experiments, risk management) becomes less incompatible and cost-prohibitive while the project is moving away from quick-to-clinic and Phase I towards a later clinical stage (9). Using risk-based tools, such as Ishikawa or control, noise, and experimental (CNX) methods for the identification of critical factors followed by failure mode effect analysis (FMEA) or risk ranking matrices for prioritization, combined with design of experiment, is an important approach to rationalizing laboratory work, better understanding method performance, and ensuring optimal project spend.
While a method cannot fail qualification, it should be ultimately scientifically sound and optimized to achieve acceptable performance capability. Developing a well-designed qualification program is therefore crucial for ensuring that the method is sufficiently robust for passing the validation step while cost incurred by the different qualification activities can be distributed across the development roadmap as a function of the level of project risk.
Finally, if third parties have been involved in the development and qualification of analytical methods, a well-designed technical transfer and appropriate documentation are required for maintaining the qualification status after the transfer of the method and to enable the validation readiness assessment exercise before ICH validation takes place.
ANALYTICAL METHOD VALIDATION AND POSTVALIDATION
Acceptance criteria must be set for validation in accordance with the ICH Q2 guideline, preferentially as a deliverable of method qualification. Therefore, all information gathered during method development and qualification is crucial for assessing validation readiness and establishing acceptance criteria in the validation protocol in accordance with process capability and product profile (see Figure 1). This compilation exercise is important in verifying that the method is ready to validate to avoid the burden of validation failures.
Figure 1: Points to consider for performing validation readiness assessment.
Once the method is ready to validate, it is strongly recommended that the ICH Q2 referential for analytical method validation is used (see Table III). The analytical validation exercise should ideally occur before pivotal studies and after clinical proof-of-concept is established for the candidate.
Table III: Analytical method validation factsheet.
Although good validation practices are described in ICH Q2, this document does not detail the practical implications for validation; for example, only a few specifics are included regarding experimental design and statistical data treatment. A clear policy is required for cGMP compliance in data acquisition and treatment, which includes developing good statistical practices. Different guidelines from the US Pharmacopeial Convention such as USP <1010> or <1033>, or from the industry such as SFSTP's (Société Française des Science et Techniques Pharmaceutiques or French Society of Pharmaceutical Science and Technology) total error describe good approaches on how to use inferential statistics and statistical intervals as well as present graphical results (e.g., using accuracy profiles) (10). Potential language gaps with the ICH, namely in the ISO definition of trueness versus accuracy, have been appropriately described to avoid misinterpretations of the validation reports. The experimental design should address all parameters from ICH Q2 following method categorization (i.e., identity, limit or quantitative impurity, potency, or active moiety). The minimal number of runs for studying accuracy and precision is best defined based on statistical t-test considerations from initial performance assessment (intermediate precision σ2) and acceptance criteria (σ) (11, 12):
These strategies meet regulatory expectations in terms of risk management of making type I/II errors as well as helping the sponsor to understand the risk-benefit of extensive experimental designs used in method validation.
A validation report is issued after the completion of the experimental plan where results are compared to acceptance criteria set in the protocol. Any nonconformity towards acceptance criteria has to be properly captured in the quality system and thoroughly investigated, preferentially using the laboratory policy for out-of-specification (OOS) investigation as background. It is intended that no broadening of acceptance criteria be decided at this stage and that a validation failure recovery plan be established. The recovery plan is typically composed of method (re)improvement and validation amendment(s). These undesirable events are, however, best prevented with sufficient prior method qualification level and adequate validation readiness assessment.
Finally, method validation cannot be seen as a discrete activity. The regulatory expectation is that the project sponsor has its own policy on postvalidation activities including method transfer and maintenance, historical trending of analytical capability, and risk assessment of changes carried out in validated methods. Good statistical practices should ensure that postvalidation activities do not alter the validated status of the method through equivalence demonstration, such as using the two one-sided t-Test (TOST), and that method performance be continuously monitored using control charts (1, 12). Postvalidation activities should be appropriately captured in the annual product quality review in accordance to ICH Q7A to provide continuous assurance that the method remains suitable for its intended use.
THE ANALYTICAL LIFECYCLE ROADMAP
With all the requirements identified and understood, a comprehensive analytical lifecycle roadmap is incorporated in the project sponsor's policy that is capable of managing the practical implications of the project (see Table IV) and staging these events across the development plan (see Figure 2).
Table IV: Practical implications of analytical method lifecycle.
Practical implications related to each step of the analytical lifecycle are then translated into defined analytical packages with regulatory-compliant deliverables staged throughout the clinical strategy (see Figure 2). These analytical packages can be used for driving the project in terms of budget and resource allocation from a phase-dependent perspective and act as yes-no decision points with respect to the general project roadmap.
Figure 2: The analytical lifecycle roadmap.
In conclusion, it is incumbent of the project sponsor to build a comprehensive roadmap that would drive the project through the different stages of clinical development in a manner that fits the economic realities of the business of developing new biologic drug candidates without compromising on regulatory compliance.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ingrid Dheur, biologics director, and Philippe Ledent, process transfer and development manager, for their insightful comments.
Pierre Douette, PhD, is head of GMP quality control at Eurogentec and contributing author to the PDA technical report on analytical method validation and transfer for biotechnology products (TR57); *Pascal Bolon, PhD, is head of sales and marketing for the biologics division at Eurogentec, Rue Bois Saint-Jean, 5, B-4102, Seraing, Belgium.
*To whom all correspondance should be addressed, p.bolon@eurogentec.com.
REFERENCES
1. S.O. Krause et al., "Analytical Method Validation and Transfer for Biotechnology Products," Technical Report 57 (Parenteral Drug Association, 2012).
2. FDA, Guidance for Industry: CGMP for Phase 1 Investigational Drugs (Rockville, MD, May 2008).
3. FDA, Guidance for Industry: INDs for Phase 2 and Phase 3 Studies—Chemistry, Manufacturing, and Controls Information (Rockville, MD, May 2003).
4. EMA, CHMP/QWP/185401/2004: Guideline on the requirements to the chemical and pharmaceutical quality documentation concerning investigational medicinal products in clinical trials (London, Oct. 2006).
5. EMA, CHMP/BWP/534898/2008: Guideline on the requirements for quality documentation concerning biological investigational medicinal products in Clinical Trials (London, Feb. 2010)
6. FDA, Guidance for Industry: Process Validation: General Principles and Practices (Rockville, MD, Jan. 2011).
7. EMA, CPMP/QWP/848/96: Note for Guidance on Process Validation (London, Sep. 2001).
8. N. Ritter et al., BioProcess Intl. 2 (8), 32-46 (2004).
9. P. Borman et al., Pharm. Tech. 31 (10) 142-152 (2007).
10. P. Hubert et al., J. Pharm. Biomed. Anal. 45 (1), 70-81 (2007).
11. USP Proposed General Chapter <1033> "Biological assay validation," Pharmacopoeial Forum,35 (2), 349-367 (2009).
12. G. Limentani et al., Anal. Chem. 75 (11), 221-226 (2005).